About Electrolytic Preparation
A fast and efficient method.
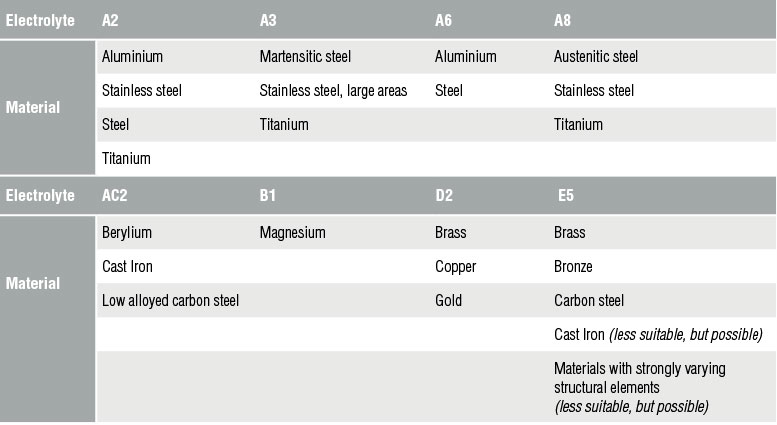
Electrolytic preparation is a fast and efficient method for metallographic preparation, providing a deformation-free surface, whereas mechanical grinding and polishing tends to leave a thin deformation layer at the surface.
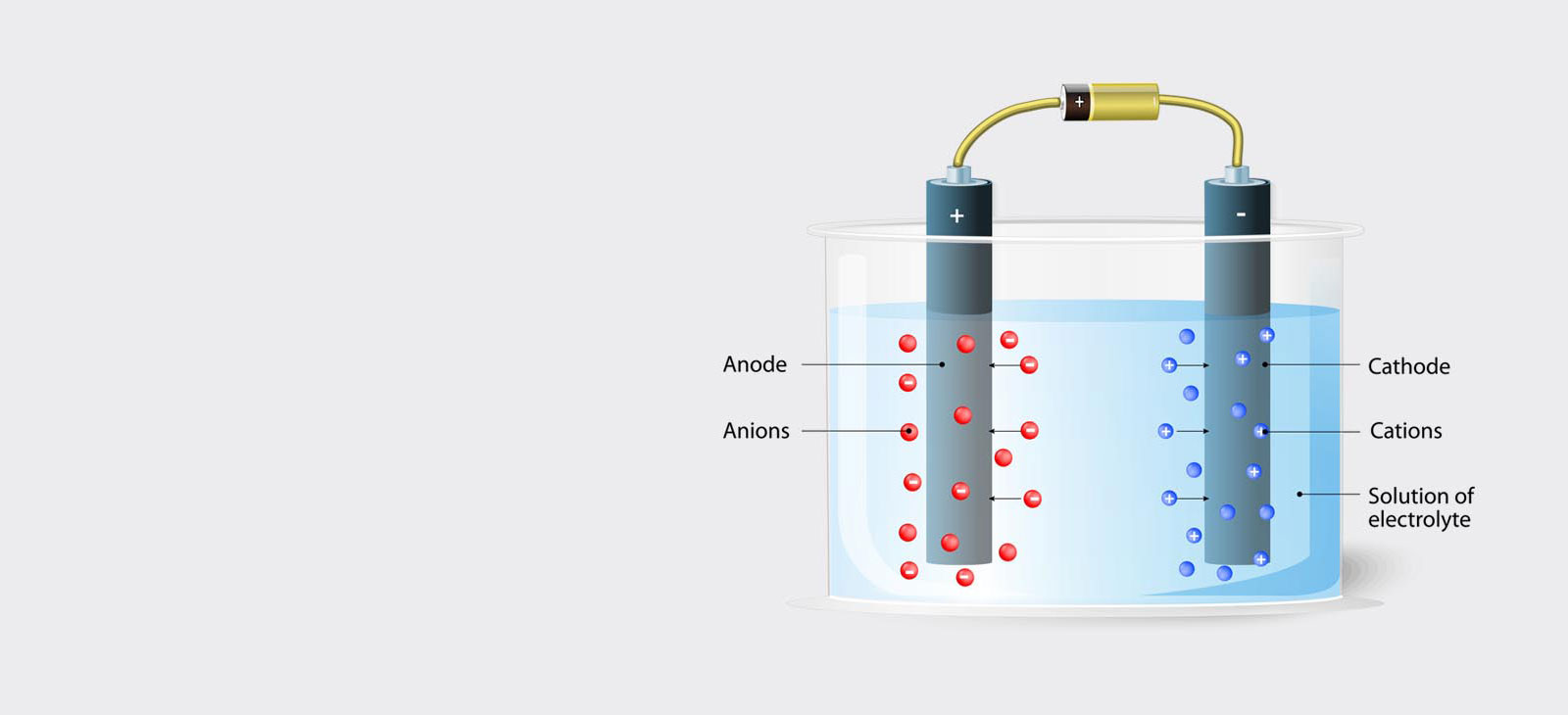
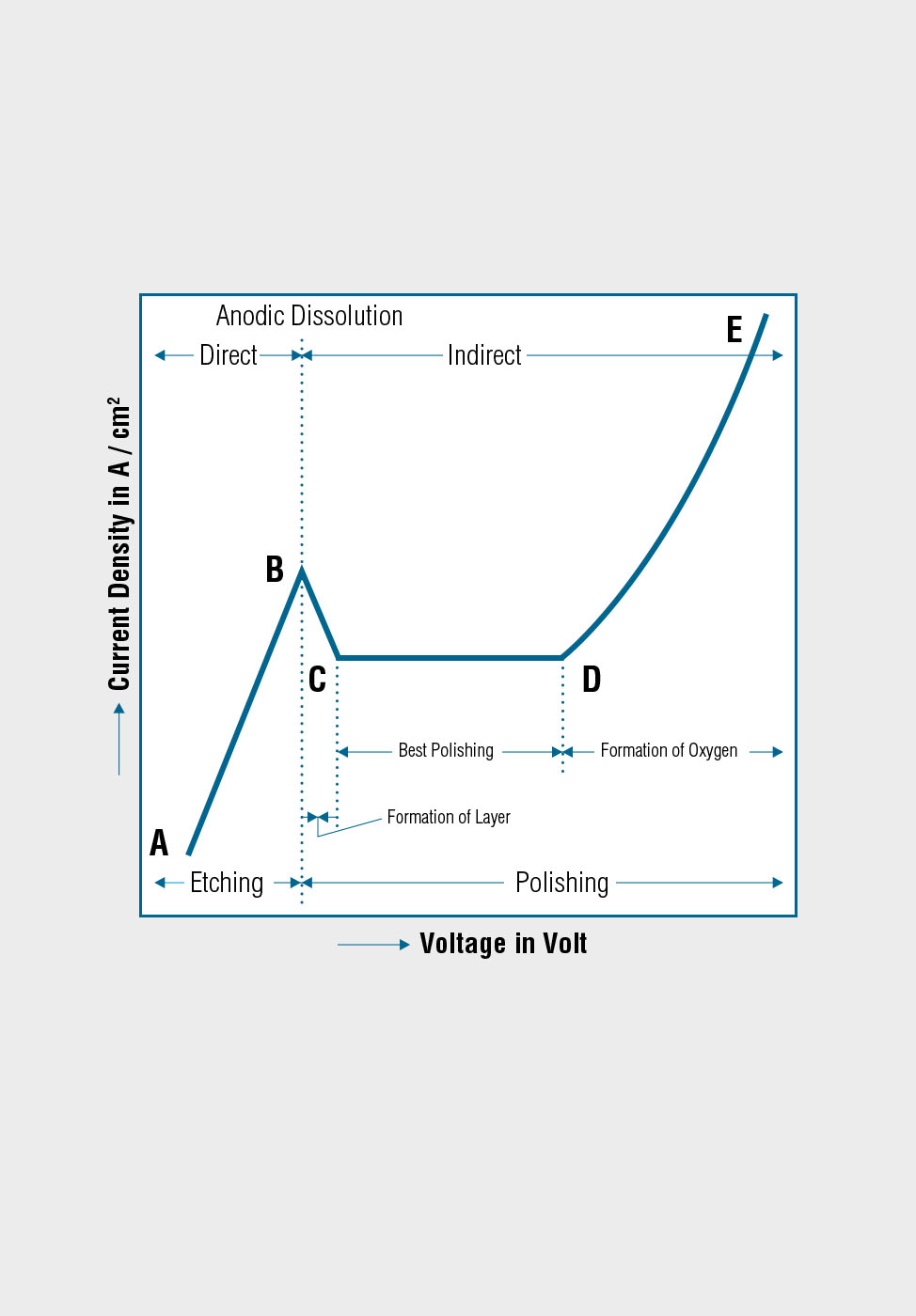
Using the electrolytic polishing equipment, the specimen is configured as the anode in a suitable electrolyte, and material is removed by controlled dissolution of the surface of the specimen. Electrolytic polishing can be followed by an electrolytic etching process to bring forth contrasts in the microstructure of the specimen.