Metallurgy and microstructure of titanium and titanium alloys
Metallography plays a key part in the quality control of titanium and its alloys, from monitoring the initial production process to assessing porosity on cast parts and controlling heat-treatment. It also plays an important part in research and development of titanium alloys and products.
Commercial titanium grades and alloys are divided into four groups:
- Unalloyed commercially pure titanium (CP)
- α and near α alloys, such as Ti-6Al-2Sn-4Zr-2Mo
- α-β alloys, such as Ti-6Al-4V
- β alloys, which have a high content of vanadium, chromium and molybdenum
At a temperature of 882°C, titanium undergoes an allotropic change from a low-temperature, close-packed hexagonal structure (α) to a body-centered cubic phase (β). This transformation makes it possible to create alloys with α, β or mixed α/β microstructures, and enables the use of heat treatment and thermo-mechanical treatment.
Consequently, a wide range of properties can be obtained from a relatively small number of alloy compositions. However, to ensure the desired combination of microstructure and properties, close control of the treatment process must be maintained. This makes metallography essential.
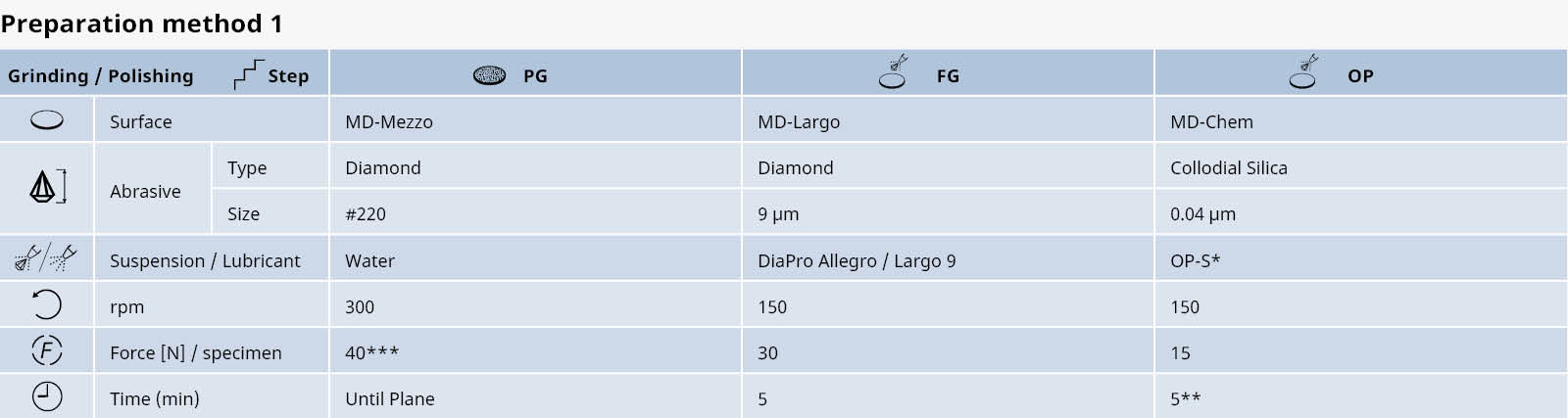
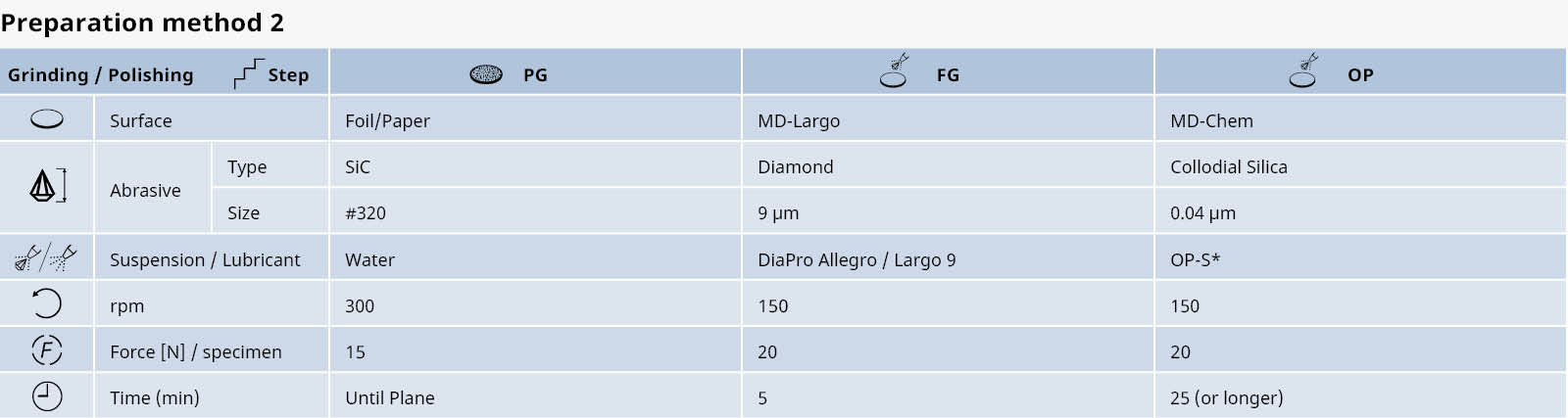
The relations between hot forming, heat treatment, microstructure and physical properties in the production of titanium and its alloys are very complex. A few examples of the most common types of titanium microstructures are shown below.
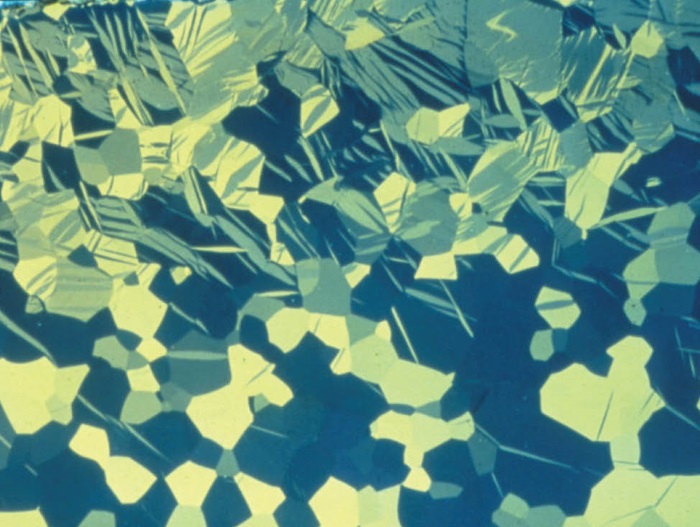
Fig. 1: Grain structure of commercially pure titanium, which has been mechanically deformed through bending. Twinning due to mechanical deformation is visible. Polarized light, 100x

Fig. 2: Structure of a forged α-β Ti-6Al-4V in the annealed condition. Etchant: Kroll’s reagent. 400x

Fig. 3: α-β Ti-6Al-4V with a white, brittle ‘α-case’ surface layer. Etchant: Weck’s reagent. Although hot forming processes are carried out under controlled atmosphere, titanium can absorb oxygen at lower temperatures, which results in a surface hardened zone, the α-case. This is a very brittle layer and can only be removed mechanically. 50x
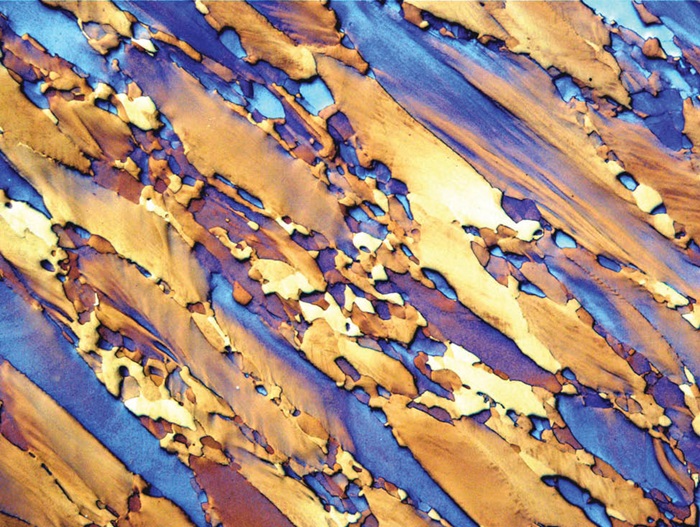
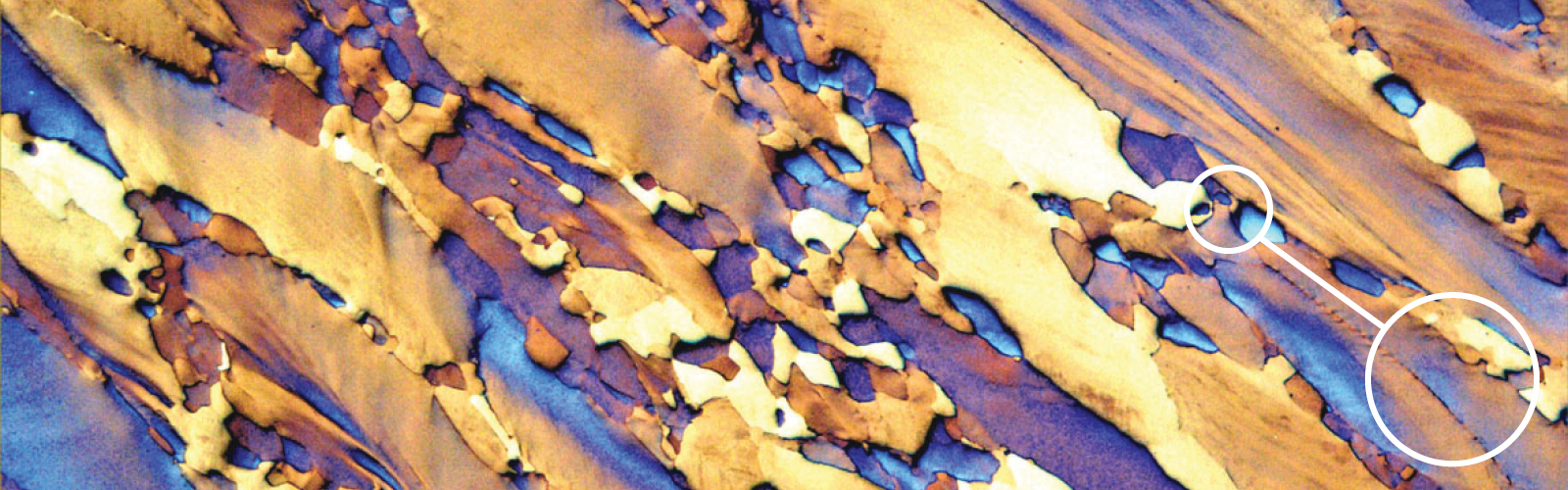
Fig. 4: The β structure of the longitudinal section of a Ti-15V-3Al- 3Sn-3Cr alloy plate. This alloy is used in the aerospace industry because of its superior mechanical properties. Etchant: heat tinting. 50x